A New Efficient Method to Prepare well-defined Functional Bioconjugates
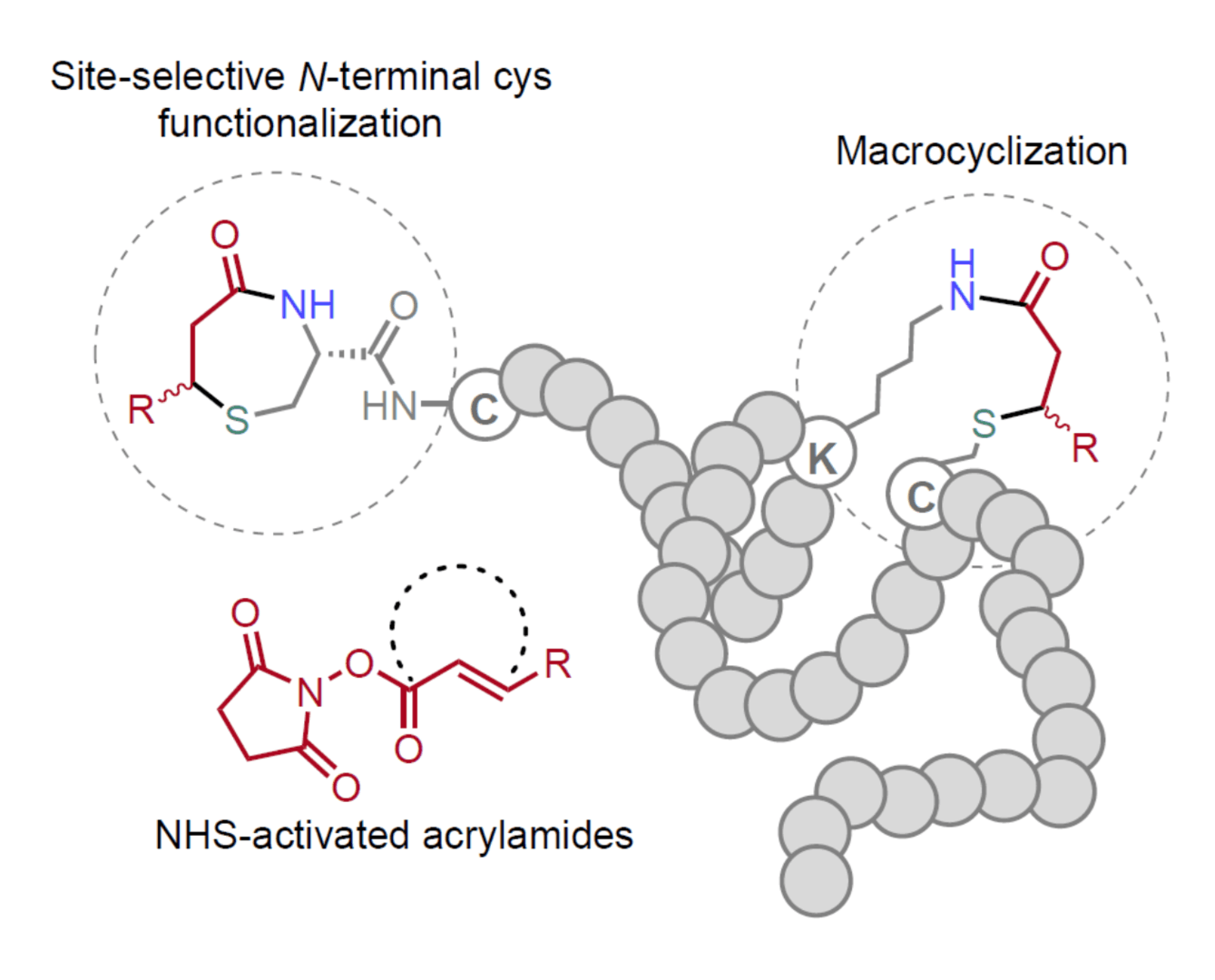
The group of Gilles Gasser at Chimie ParisTech – PSL, CNRS, Institute of Chemistry for Life and Sciences in collaboration with Pedro Góis at iMed.Ulisboa, Faculty of Pharmacy, University of Lisbon in collaboration with, Adelaide Fernandes also from iMed.Ulisboa, Faculty of Pharmacy, University of Lisbon and Francisco Corzana at Departamento de Química, Centro de Investigación en Síntesis Química, Universidad de La Rioja have described the rational design of NHS-activated acrylamides for the efficient chemoselective amino-sulfhydryl stapling on native peptides and proteins.
This work was recently published in Angewandte Chemie International Edition and will contribute to the extension of the chemical biology toolbox for the design of well-defined functional bioconjugates.
In the last decades, bioconjugation strategies have become valuable for pharmacokinetics improvement using targeting delivery systems such as peptides and antibody drug conjugates (ADC). Those developments have also been applied for targeted bioimaging probes, advanced peptide structural studies to tackle difficult targets as protein-protein interaction, among others. Usually, the major challenges in this field is the design of bioconjugation reagents that provide site-selective and orthogonal modifications in complex biomolecules. Besides this, the bioconjugation reactions should proceed under physiological-like conditions.
In this recent article, the authors used NHS-activated acrylamides for site-selective modification of N-terminal cysteines in peptides with fast kinetics and under dilute aqueous conditions. Additionally, these novel bioconjugation handles allow the macrocyclization between an in-chain or C-terminal cysteine with a lysine side chain close-by. The versatility of this strategy for orthogonal dual-modification was achieved thanks to its compatibility with other common cysteine selective reagents and was also useful for late-stage design of functional bioconjugates equipped with different payloads.
Professor Gilles Gasser from Chimie ParisTech – PSL said: “This discovery can tremendous applications in medicinal chemistry. I am very much looking forward to using this technique to deliver some of our compounds specifically to tumours”.
Maria José Silva, currently working as exchange PhD student at Chimie ParisTech – PSL in the group of Gilles Gasser, said: “It is very gratifying to be part of such a multidisciplinary project on a new bioconjugation strategy with high level of control and versatility.”
